Scientists mapped the hybrid cells that may lead to metastasis
A group of researchers from the University of Milan drew a map of the transitions that can lead tumour cells to become metastatic. Such an outcome provides useful information about the characteristics of cells during these transitions and allows researchers to visualise the activity of the genes involved. The authors of the study are part of the Centre for Complexity and Biosystems (CC&B) and their research was published on PNAS.
Our skin, as well as the inner and outer surfaces of our organs, are made of epithelial cells, which are closely connected to each other and pressed together in adherent layers that restrain their mobility. In some cases, this kind of cells can lose their features and transform into mesenchymal cells, which are less connected among them and can migrate easily. This happens during embryo development, when mesenchymal stem cells may then differentiate into bone, muscle, cartilage and fat cells, or during wound healing. But such a process is also particularly relevant for cancer: almost 80% of human malignant tumours originates from epithelial cells that have become extremely aggressive and have invaded other tissues.
“We know from previous studies that the transition from epithelial to mesenchymal cells is a complex process, during which cells pass through intermediate states with mixed traits,” explains Francesc Font-Clos, post-doc researcher at the CC&B and first author of the study. “For instance, they can combine a high mobility with adherent properties, which allow them to easily invade other tissues and then colonize them”.
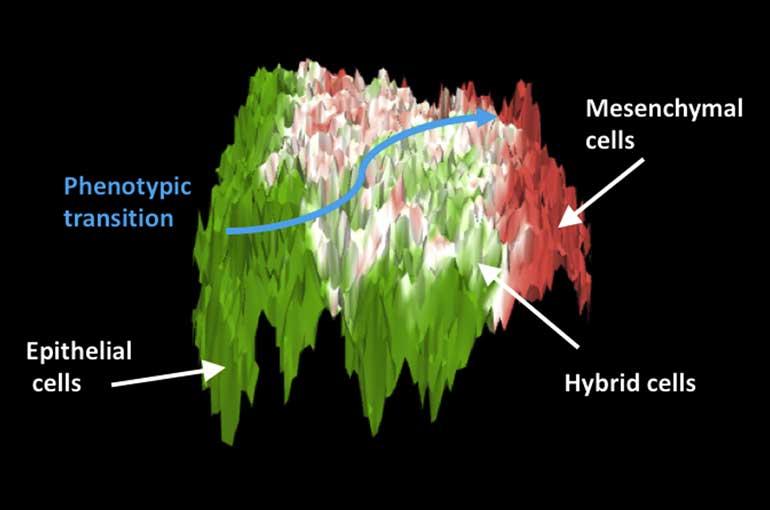
Cells with these hybrid traits are often unstable and their variations are determined by several genetic, physical and environmental factors. To study such a complex biological network, researchers from the CC&B used a mathematical model to simulate the transition from epithelial to mesenchymal cells, including the intermediate states. By doing so, they managed to produce a two-dimensional map that represents this transition as a rugged landscape with fractal-like features: different kinds of cells have their own positions in this landscape.
The researchers then analysed the expression of large set of genes from different kind of tissues, finding that the map obtained from experimental data is similar to the one simulated with their model. They located the genetic signatures of epithelial and mesenchymal cells, which represent the two stable and well-defined extremes of the transition; between them, they observed several hybrid states, with their own genetic characteristics, that are particularly prone to external perturbations. This strong kind of plasticity is often associated with the highly aggressive behaviour of tumour cells.
“It is also interesting to note that the fractal-like traits of our landscape are similar to those that can be observed in studies on disordered solids and glassy materials, showing that there are some common physical traits in the transition dynamics in organic and inorganic systems”, said Stefano Zapperi, professor at the Department of Physics, who also contributed to the work.
The methodology developed by the CC&B researchers is not just a way to visualise the possible conformations assumed by those hybrid cells that could become malignant, but may also provide the possibility to measure the gene activity correlated to these intermediate states and facilitate the analysis of large set of sequencing data.
“Tumours are heterogeneous and the analysis of single cells that we carried out in this study sheds new light on the cancer development”, concluded Caterina La Porta, professor of General Pathology at the Department of Environmental Sciences and Policy of the University of Milan and coordinator of the research. “In particular, it helps us to understand the appearance of metastasis. In fact, we know that metastasis could come out after many years and in sites not related to the primary tumour. With our approach, we could literally see how this transformation occurs and describe a new method using network science on single cells to detangle tumour heterogeneity in view of personalized medicine”.
Paper reference
Topography of epithelial-mesenchymal plasticity
PNAS, Francesc Font-Clos, Stefano Zapperi and Caterina A. M. La Porta
PNAS (2018) http://www.pnas.org/content/early/2018/05/16/1722609115


